Page 4 of 13
Design development planning
What is a Design Development Plan?
The design and development plan (DDP) is our primary planning tool and a crucial part of thinking about a product and setting up a new project to deliver it. We use it to make sure we’ve thought about the end user and the steps that need to be taken to deliver to their needs while taking account of risks and ethics.
The DDP is the responsibility of the project lead, bringing in whatever expertise and knowledge they need to complete each part.
It can be tempting to skip this and dive right into a project before the DDP is fully completed but this inevitably leads to problems later on: a risk that could have been foreseen and prevented, or a problem caused by a lack of clarity around people’s roles, for example.
The DDP includes the following main areas:
- Project overview: key parameters, milestones, roles and scope of project
- Use and market targets: who will be using this and how
- Project plan: how the project will be managed
- Roles and responsibilities: personnel and skills needed
- Supplier agreements and subcontracts. what external skills and resources might be needed
- Deliverables: what needs to be produced (this might be software, a product, study data etc) and timings for this
- Study management: how the research phases will be managed
- Risk management: how will risks be analysed, recorded and evaluated
- Software development: full details of any software that might need to be produced, including verification and validation for this
- Verification planning: how you will ensure that what you are building does all the things you said it should
- Validation planning: how you will check that you are building the right thing
- Design reviews: how often should these be and who needs to be involved
- Data management: what data will be collected and how this needs to be stored including details on data integrity.
- Issue resolution and change control: what happens if changes are needed or problems arise
- Data retention: how long will data be stored, and how
- Document control: how documentation for the project will be managed
- Analysis: defining the approach to any data analysis that is required
- Client communication: how will the client be kept up to date and who is the point of contact
Adapting a DDP to your project:
- Every project is different, the DDP is adaptable to the needs of a specific project so if some areas are genuinely unnecessary you can leave them out.
- This document can form part of the planning for both Agile and Waterfall project management styles. Even if a project will be iterative and run in sprints it still needs this level of planning at the beginning.
- Finally, the DDP isn’t something that gets ignored after the project starts, it’s a working document that should be revisited and updated on an ongoing basis, at the very least at the end of each design and development milestone.
Resources
Design Development Plan TemplateTake a look at our DDP template document which outlines in more detail the information that should be included in each section.
First we want to take a look at user and market targets, outline an approach to risk and also think about data management. In the next section we’ll go into more detail on project planning and roles and responsibilities.
Focus area: user and market targets
Defining your audience should be a priority at the beginning of a project. This will allow you to appropriately tailor the end product, test it with the right people and deliver an effective marketing plan (we will look at this in more detail in Module 2).
Note: “everybody” is not a valid target user group! Even projects with broad potential appeal should identify the priority user group.
This doesn’t need to be a long definition. For example: when we worked on a project around insomnia, our definition in the DDP was:
People with a clinical diagnosis of insomnia who meet the following criteria:
Initial Project
Working with dedicated medical recruiters in the US and Germany, Ctrl Group plan to recruit 15 participants in each study site. This is to allow for attrition and ensure we can start the study and gain insights from at least 12 participants per country.
We will recruit and seek consent in line with the following criteria:
Inclusion criteria
- Must have been diagnosed with impaired sleep patterns for 3-4 nights per week for the past 3 months
- Using medication or alternative treatments to treat insomnia (This information will be captured in recruitment screening)
- Smartphone user: iOS or Android (To assure representative recruitment from participants over 65 years old, we’ll also offer a paper-based option for those without mobile phones or tablets)
Primary segmentation for recruitment
- Country: US (N=15), DE (N=15)
- Age: 18-65 years old (N=15), 65-85 years old (N=15)
- Symptoms: Difficulty to fall asleep > 20 mins (N=15); Waking after falling asleep > 30mins (N=15)
Secondary segmentation for recruitment
- Best efforts to get coverage across gender, socioeconomic status and age range within segment
Focus area: risk management
Identifying risks and how to mitigate them is a fundamental part of project planning. This process highlights possible issues and gives you a chance to plan for them or head them off before they become a problem. It also functions as a way to expose assumptions and make sure that everyone is in agreement and communicating clearly.
For health care related projects this is especially important as you’re dealing with sensitive areas that may even be a matter of life and death, so the risk tends to be high. If your study relies on ethics approval from an institution, evaluation of risk will be a requirement.
It can seem a bit like trying to predict the future, but gets easier once you have been through the process a few times and can base the risks on previous experience.
Management of risk is key to the ISO-13485 process which states ‘The organization shall establish documented requirements for risk management throughout product realization. Records arising from risk management shall be maintained’. There is also separate guidance for risk management in the form of ISO-14971.
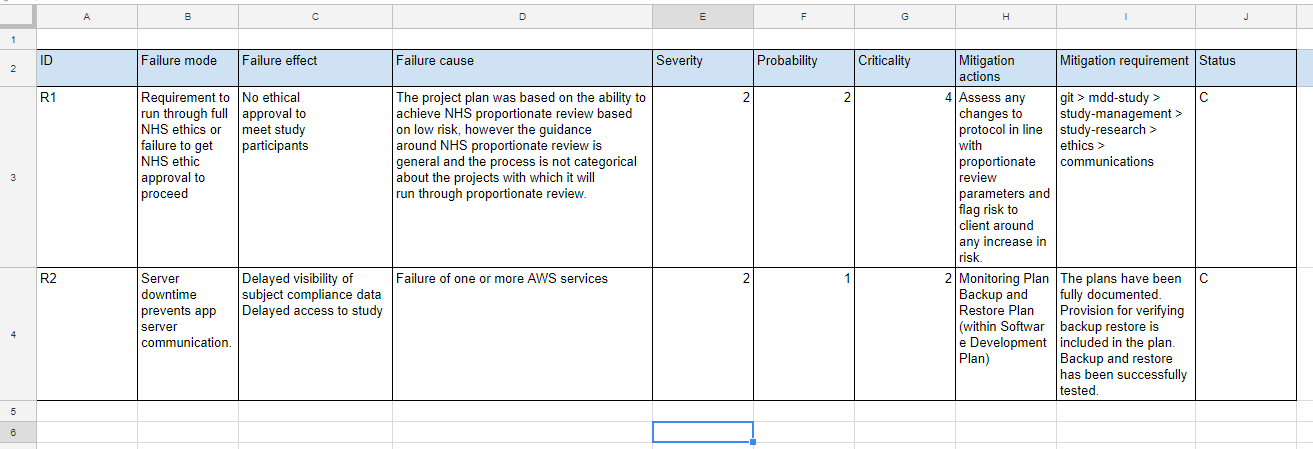
At Ctrl Group we use the following format to complete a risk register – a spreadsheet that logs and tracks potential risks associated with the project and the product use:
Risk register format:
- Failure mode: A short readable sentence describing what could go wrong.
- Failure effect: A short readable sentence describing the effect of the failure.
- Failure Cause: A short readable sentence describing the cause of the failure.
- Severity: [1 Insignificant, 2, 3, 4, 5 Critical].
- Probability: [1 Rarely, 2, 3, 4, 5 Certain].
- Criticality: [Severity, × , Probability].
- Mitigation actions: A list of actions to be taken. These should be determined during a design review meeting.
- Mitigation requirement: A reference to the requirement where the mitigation actions will be tracked.
- Status:
- [I] Identified - risk identified in initial risk analysis and discovery.
- [A] Active - risk has occurred and needs to be managed.
- [C] Closed - risk has occurred and managed.
An example of this is below:
Focus area: Data Management (and Data Integrity)
In our Design Development planning document you’ll see that Data Integrity is a subsection of Data Management. But what does this mean?
As a design research company, data is at the heart of everyday working practice at Ctrl Group. Through the design and development of clinical research Ctrl Group record, collect, transpose, manipulate and transform data through various methods.
The data lifecycle at Ctrl Group refers to the journey data takes from being generated and collected through to completion of a project or work relationship. Data integrity in this context is the need for Ctrl Group to ensure that the lifecycle of data is supported in its accuracy and consistency. Data integrity arrangements must ensure that the accuracy, completeness, content and meaning of data is retained throughout the data lifecycle.
Data should be ALCOA:
- Attributable
- Legible
- Contemporaneous
- Original
- Accurate
Regular reviews of the data are also a crucial part of our process and are completed by the Project or Research Lead as part of their project management responsibilities. Periodic reviews are completed by the quality manager auditing all company data and the operational and technical measures.
Example Situation
A recruiter was in a rush and noted some of the participant data incorrectly and has not noticed. We’ve received this data.
Question: How do we confirm that data is correct and rectify the error?
Response:
- The research team follows specific questions at the beginning of their first contact with the participant, which includes confirming their personal information. This is associated with their participant ID
- Upon learning the information is incorrect, the research team member amends the data, logging an incident and the corrective actions taken in the risk register
- Request that the recruiter re-confirms the details with the participant - ensuring that no specific details of the information are released
- Once the updated details are received in the password protected document, they are cross checked with updated information

Case study
Launching an application to help track cognition and mood in depressed patients using wearable technology
Challenge
Working in partnership with distributed teams in London and Cambridge in the UK and in several US cities, we planned out a project that would not only clarify the roles and timelines but consider the risks and ethics at all stages.
Approach
- Iterative design and development with visibility for all parties using a shared repository
- Decision to assess the prototype both internally and then through a clinical trial, which we would manage
- Specification of all details of data management, software development and analysis in advance
- Opened up our internal processes - our quality management system - for external review against good clinical practices and industry standards for IT and Privacy/Security
- Decision to seek ethical approval through NHS proportionate review
- Recruitment of users through a third party medical recruiter
Results
- External reviews of the quality of our approach and of our internal processes gave us confidence in our processes. It also helped us identify processes we felt could be improved.
- NHS Ethics proportionate review was granted for Ctrl Group to run the clinical trial
- Trial completed and clinical study report created, showing results of the study
- Press release published, outlining study findings
- Findings used to validate the product features and stimulate ongoing product development