Page 8 of 13
Documenting design decisions
Why do you need to document design decisions?
When making medical technology, the requirements for documenting the design process are more stringent than in other design projects, as these products have the potential to contribute to the death, serious illness or injury of a user. The design history file (DHF) was introduced in 1990 as part of regulation passed by US Congress, and is a way of doing this. So what is it?
Design history file (DHF)
When you design something you go through a process of making decisions about why it should be a certain way. For instance, you might test your design with people and find the design doesn’t fit the requirements. Taking this into account, in the next iteration of your design you would make a number of changes to better fit the requirements.
This diagram shows an idealised version of this process.
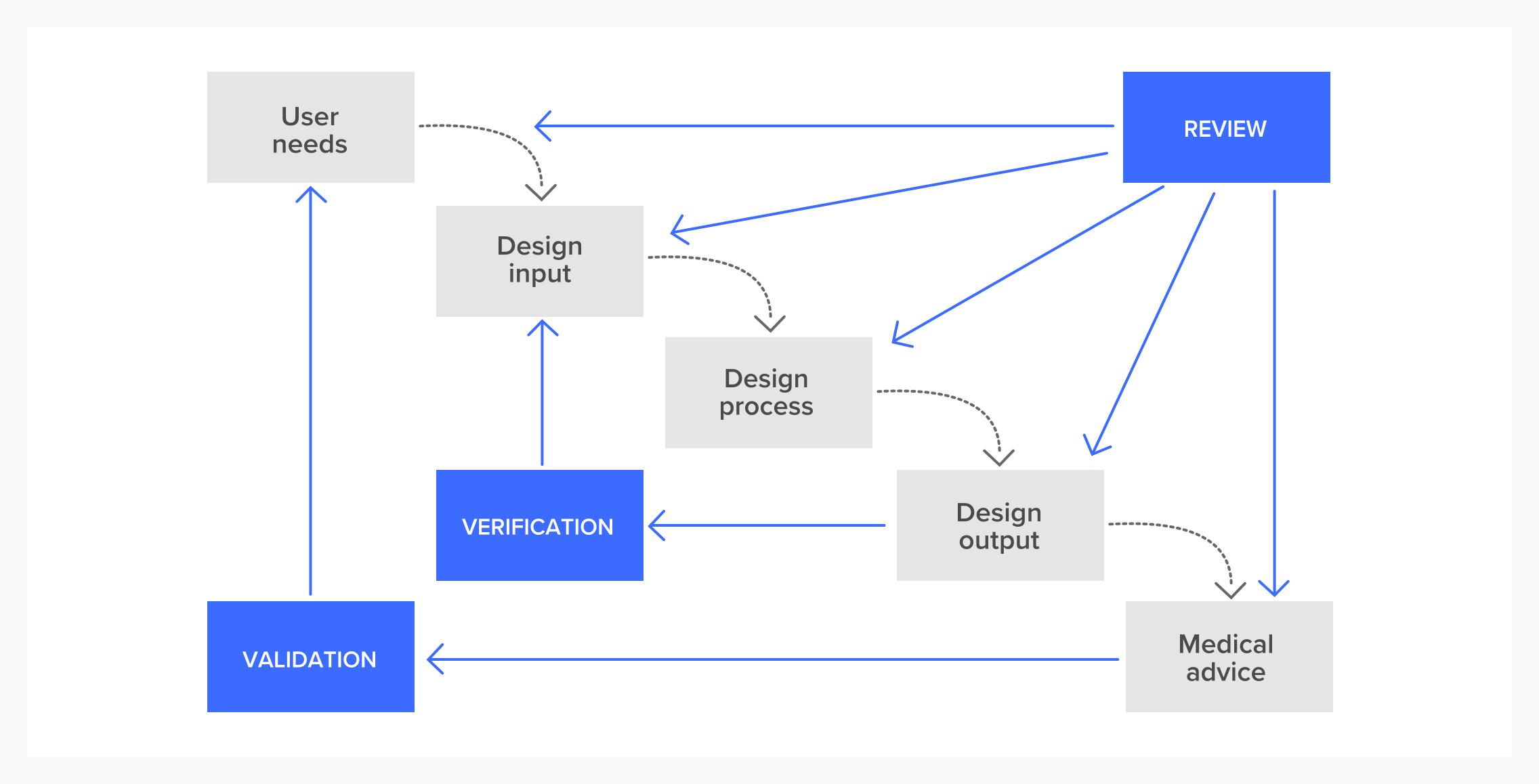
You could almost think of this list of decisions as the story of your design. Along the way you will be constantly iterating: validating and verifying your decisions.
As you will have already seen in our Design Development Plan, we suggest having a plan for how you’ll your project from the outset.
Definitions
Validation: are we building the right thing?
Verification: does this thing we’ve built do all the things we said it should?
A DHF captures this story. It is the record of all the decisions you made along the way, and why. It is likely to be a group of documents, rather than just one (see below for tools you can use).
In the design of medical devices or software there are regulatory processes that mean you’ll often need to justify the design of a device or software. For instance, if you were creating a medical device and eventually wanted to distribute for use in the US, the device will need to be submitted to the Food and Drug Administration (FDA) for approval. As part of this submission you would need to provide a DHF. You may also need to provide this as part of the auditing process for other national and international regulations and standards.
Elements of a design history file
The key elements that make up your story:
- The starting point (called requirements in DHF)
- Requirements
- User Needs and Plan
- Your design (called design output in DHF)
- Snapshots of your design
- Feedback and reviews (called design reviews and validation in DHF)
- Research Insights
- Meeting Minutes
- Arriving at a final design (called verification in DHF)
- Verification Report
Other important elements might be if the requirements for the design changed for some reason, as this would mean the design would also need to change. It’s fine for requirements to change, but the change needs to be recorded.
In our experience, it’s much easier to integrate the recording of the story into our workflow than to try and construct it retrospectively. The risk is that the process of documenting while working takes a lot of time. We try and use lightweight digital tools that help us to record this history while maintaining our momentum and agility.
Tools for creating a design history
Any tools that help you record a chronology of events and link to other resources will be useful in recording your design history.
For instance, Git is a tool used widely for code management in software development. It can also be used to track changes in any set of files. You could also use any or a combination of these:
- A versioned wiki
- Google Docs
- Airtable
Resources
Design history file.
Wikipedia entry on the Design History File
Software as a Medical Device.
FDA advice on Software as a Medical Advice