Page 2 of 14
Using research throughout the product development cycle
Module 2 focused on using research to understand user needs and inform the design process. While the techniques used in Module 2 are often used at the initial stages of product development (so that you start with a good understanding of user needs), they can also be used throughout the development process.
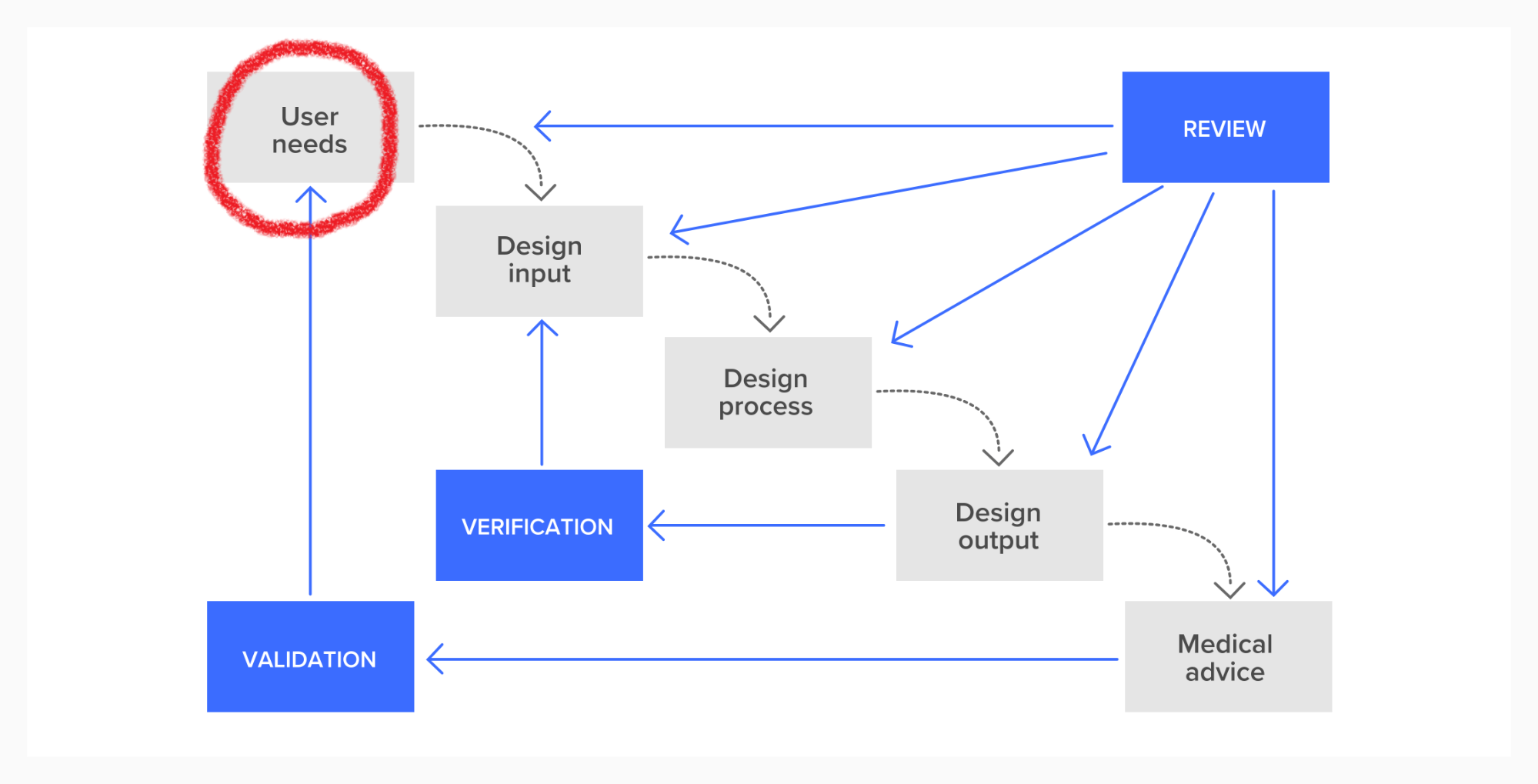 Module 2 looked at user needs, Module 4 looks at the rest of this process
Module 2 looked at user needs, Module 4 looks at the rest of this process
However, there are a number of additional approaches that are useful to verify and validate your product for launch and in market. While validation can and should happen during initial phases of the project, it becomes increasingly vital as the fidelity of your designs increase.
This is what we’ll be looking at in this module - what happens when you have a clearer view of user needs and moving into a process of verification and validation, before finally launching your product in the market.
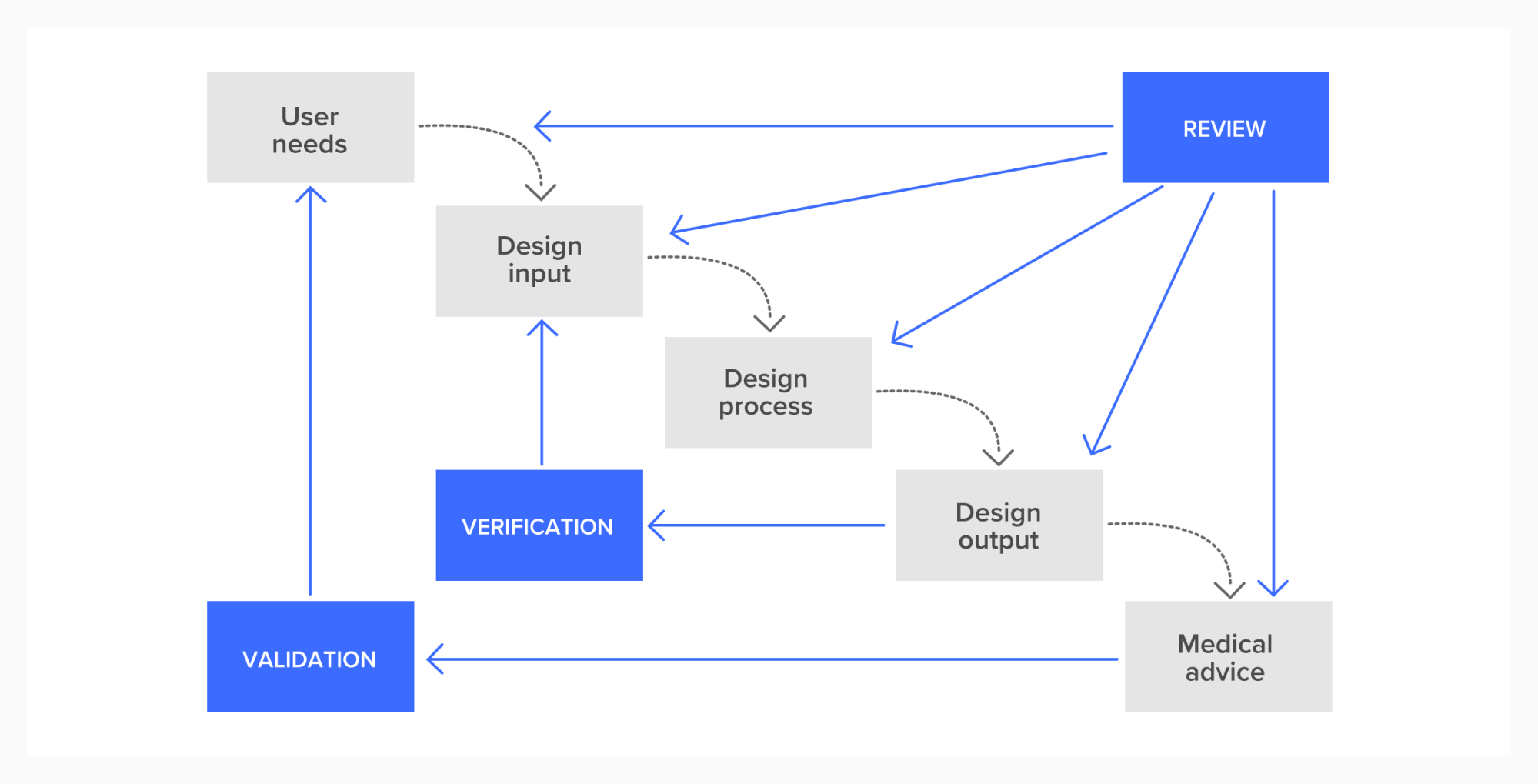
Key terms
Design verification: This means confirming that your design outputs (e.g. high-fidelity prototype, service designs, processes etc.) conform to design inputs (e.g. specified user requirements). In other words, checking that what you’re making still meets the requirements in theory.
Design validation: This means confirming that the resultant deliverable design meets its intended purpose in the specified context(s) of use. Put simply, checking that what you’re making works in practice.
Design Transfer and Manufacture: Once you’ve reached the end of this process, and have your final designs, you’re ready to turn them into manufacturing specifications. Once the design transfer files (e.g. requirements, deliverable design, drawings, software design) are ready they’re transferred to manufacturing. Here, design inputs are considered final.
Real World Evidence (RWE): This is information about the use of your product collected from various sources once it’s launched and being used by patients. It may come from medical records, complaints, reviews or analytics, for example. This might not be a controlled trial, but it can still be very useful for further development and for flagging issues.
Techniques for verification and validation and factors to consider
Some of the research techniques described in Module 2 can also be used here. However, the process becomes more formalised now that you’re trialling the product with users and there are therefore more stringent guidelines and reporting requirements to follow. Additional techniques and factors to consider:
Human factors documentation: There’s a requirement to have full documentation available for potential submission to regulatory bodies.
Good Clinical Practice: Good clinical practice (GCP) is the international ethical, scientific and practical standard to which all clinical research is conducted. Compliance with GCP provides public assurance that the rights, safety and wellbeing of research participants are protected and that the research data is reliable.
Adverse events and incidents: These are unfavourable or harmful effects or problems that occur during the trial of a health product, service or drug. We collect reports of adverse events and incidents in order to continually monitor the safety profile of Ctrl Group products and services.
Additional techniques used at this stage include:
- Usability evaluation
- Longitudinal evaluation
- Clinical evaluation
- Real World Data and Evidence
We’ll be looking at these in the following sections.